
In 2008, TMS was FDA-approved to treat major depressive disorder that did not resolve with antidepressants. The major clinical trial included 20-30 treatments, each of 38 minutes duration. It was sham-controlled and included about 300 people, and the reuslts showed about a 200% increase in resolution from depression in the active TMS group over sham. This was staggering data — light years ahead of the results achieved from antidepressants. It was also a very well tolerated, meaning minimal potential for side effects.
Since then, TMS has been studied by universities and research centers word wide. A multitude of new TMS devices and TMS protocols have been tested and FDA-cleared. Protocols treating different areas of the brain have also been found to be effective, not just for major depression, but also for anxiety disorders, substance use disorders, ADHD, autism-spectrum disorder, and even dementia.
In 2018, another breakthrough occurred, a TMS protocol called intermittent theta burst (iTBS), which is three minutes in duration. In a study of 414 people, iTBI (3 min) was found to be equivalent to the standard 38-minute protocol. It was subsequently FDA-cleared. The total treatment time was four weeks (one treatment daily, M-F, for four weeks)
Both of these protocols were found to yield the following results:
30-40% remission (resolution or near resolution of depression symptoms)
20-30% response (over 50% improvement in depression symptoms)
In total, about 50-60% of people who underwent TMS treatment had drastic improvement.
In 2022, a research group from Stanford University tested a protocol that decreased the length of the treatment from six or seven weeks to one week. It was called accelerated TMS or the SAINT protocol. The protocol involved delivering three continuous iTBS treatments (10 minutes duration), every hour for ten hours, for five consecutive days. It increased the total amount of treatment delivered in the prior iTBS protocol by around five-fold.
After four weeks, the SAINT protocol yielded the following results:
78% remission (resolution or near resolution of depression symptoms)
7% response (over 50% improvement of depression symptoms)
These results were staggering. Excitement about the potential for the SAINT protocol spread like wildfire within the psychiatric community. The main reason for the level of excitement that ensued was the magnitude of the results, particularly in a patient population who had tried, on average, depression for over 20 years, had tried eight different antidepressants, with some having also tried TMS, all without success.
The other reason was that standard TMS, which requires one daily treatment for about 6-7 weeks, is logistically a major hassle. It is common that people are unable to come to an office daily due to their jobs or other responsibilities. Therefore, the SAINT protocol was a refreshing upgrade.
But one issue that tempered this excitement was the durability. The benefit seemed to taper off in the months after completing the treatment. A positive finding to counter this issue was that retreatment with the SAINT protocol was found to yield essentially the same.
The SAINT protocol was FDA-approved in late 2022 but, to date, commercial health insurance does not cover the treatment.
As a result of this remarkable research, many institutions have tested accelerated TMS protocols, using a smaller total number of treatments, different TMS parameters, and with different TMS equipment.
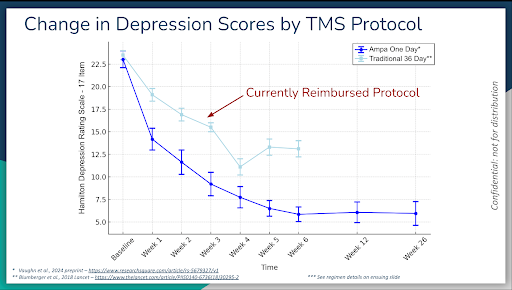
In 2025, another accelerated protocol was tested in a group of 32 people with depression, called ONE-D. This time, the protocol involved one full-day of treatment only, with a total of 20 iTBS treatments throughout the day. It also included administering two medications, one dose each, for only one day. One of the medications was D-cycloserine, an antibiotic that is thought to promote brain rewiring, with the other being a psychostimulant called lisdesamphetamine (or Vyvanse).
After six weeks, ONE-D yielded the following results:
74% remission (resolution or near resolution of depression symptoms)
16% response (over 50% improvement of depression symptoms)
Symptoms of anxiety were also assessed with a tool called GAD-7, and ONE-D yielded the following results:
76% remission (resolution or near resolution of anxiety symptoms)
17% response (over 50% improvement in anxiety symptoms)
It is important to note that this data was released by the researchers before being published in a peer-reviewed journal. Nonetheless, excitement reached SAINT-level proportions. The main question that remained to be answered was, “How long does the benefit last for?”
To answer this question, six-month follow-up data was released:
What was revealed was this: One day of this TMS treatment program, along with one dose of two medications administered on the day of treatment, yielded drastic benefits that lasted for six months. We eagerly await 9 and 12 month data.
ONE-D has become a treatment option for those who are unable to commit to 7 weeks of standard TMS treatment. Although one major issue is the lack of insurance coverage for this treatment. The problem with the latter is that technological advancements in TMS, and psychiatry in general, have outpaced our ability to achieve FDA-approval in a timely manner, and far outpaced Federal and commercial health insurance coverage determinations.
The field of psychiatry has, over the years, used TMS earlier and earlier in the course of treatment for depression. The old method of treating depression and anxiety was that we would try an antidepressant, increase the dose, wait two or three months, and if it didn’t work, taper off the first antidepressant and try another antidepressant. Rinse and repeat until we are successful.
The problem with this strategy is that after two antidepressants, the chances of having success with the third is about 10%, and even then the effect is unlikely to hold at the three-month mark, even for the lucky 10%. Therefore, TMS found its place as step 3, after two unsuccessful antidepressants. Some would argue that it could take the place of antidepressants all together.
